Home page Description:
Study reveals how cells are activated to clear harmful pathogens and debris in the brain.
Posted On: June 01, 2016
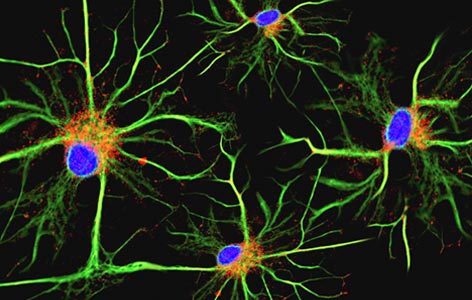
Image Caption:
Microglial cells (pictured using immunofluorescence microscopy) form the main immune defense system in the brain and spinal cord and account for around 10–15% of all brain cells.
Microglial cells (microglia) are the brain and spine’s first line of defense: they clear unwanted pathogens and cellular debris—which can prevent new tissue growth and promote more damage—through a process called phagocytosis. This process, however, also may produce reactive oxygen species (ROS) that can damage surrounding cells.
The amount of ROS that is created depends on the microglial cells’ ‘activation state’. It is believed that when microglia are in an ‘anti-inflammatory activation state’, they support tissue repair; however, when they are in a ‘pro-inflammatory activation state’, ROS levels are high and unwanted tissue damage can occur. When the inflammatory balance shifts towards the pro-inflammatory state, microglia can cause chronic damage that can lead to neurological diseases such as Parkinson disease.
Despite the critical difference between pro- and anti-inflammatory behaviours, very little is known about how microglia cells change between activation states.
Krembil Senior Scientist Dr. Lyanne Schlichter and her team explored this balance in microglia activation state by exposing the cells to various factors—known as pro- or anti-inflammatory cytokines—in the presence or absence of debris from damaged neurons. By observing the cells under different regimens, they were able to identify how different conditions affect microglial cell properties, including whether they produced ROS or carried out phagocytosis.
“If we can determine how to influence microglial activation states to promote beneficial effects of phagocytosis while suppressing damaging effects of ROS, then we can help develop therapeutic interventions to reduce neurodegeneration and to promote cellular recovery following brain damage,” says Dr. Schlichter.
This work was supported by the Canadian Institutes of Health Research, the Heart and Stroke Foundation of Canada and the Toronto General & Western Hospital Foundation.
Complex molecular and functional outcomes of single versus sequential cytokine stimulation of rat microglia. Siddiqui TA, Lively S, Schlichter LC. Journal of Neuroinflammation. doi: 10.1186/s12974-016-0531-9. 2016 Mar 24. [Pubmed abstract]
The amount of ROS that is created depends on the microglial cells’ ‘activation state’. It is believed that when microglia are in an ‘anti-inflammatory activation state’, they support tissue repair; however, when they are in a ‘pro-inflammatory activation state’, ROS levels are high and unwanted tissue damage can occur. When the inflammatory balance shifts towards the pro-inflammatory state, microglia can cause chronic damage that can lead to neurological diseases such as Parkinson disease.
Despite the critical difference between pro- and anti-inflammatory behaviours, very little is known about how microglia cells change between activation states.
Krembil Senior Scientist Dr. Lyanne Schlichter and her team explored this balance in microglia activation state by exposing the cells to various factors—known as pro- or anti-inflammatory cytokines—in the presence or absence of debris from damaged neurons. By observing the cells under different regimens, they were able to identify how different conditions affect microglial cell properties, including whether they produced ROS or carried out phagocytosis.
“If we can determine how to influence microglial activation states to promote beneficial effects of phagocytosis while suppressing damaging effects of ROS, then we can help develop therapeutic interventions to reduce neurodegeneration and to promote cellular recovery following brain damage,” says Dr. Schlichter.
This work was supported by the Canadian Institutes of Health Research, the Heart and Stroke Foundation of Canada and the Toronto General & Western Hospital Foundation.
Complex molecular and functional outcomes of single versus sequential cytokine stimulation of rat microglia. Siddiqui TA, Lively S, Schlichter LC. Journal of Neuroinflammation. doi: 10.1186/s12974-016-0531-9. 2016 Mar 24. [Pubmed abstract]




