
Researchers at UHN’s Donald K. Johnson Eye Institute (DKJEI) have identified key processes that guide the positioning of light-sensitive cells in the retina—the neural tissue that lines the back of the eye.
All brain functions, from thinking and feeling to controlling the body’s movements, hinge on precise communication between neurons. To communicate effectively, neurons must migrate to particular positions and connect with nearby cells to form specialized circuits.
A fundamental question in neuroscience is how neurons find their place and form neural circuits—and how this process goes astray, leading to disease.
In a study published in Proceedings of the National Academy of Sciences, researchers led by DKJEI Co-Director and Senior Scientist Dr. Valerie Wallace explored the mechanisms underlying neural circuit formation in the retina.
“The human brain has billions of neurons, so it is extremely challenging to study, at the single-cell level, how circuits form and change over time,” explains Dr. Wallace. “The retina, an extension of the brain, has a much simpler structure, wherein different cell types reside in distinct layers. This arrangement makes it easy to pinpoint when cells are out of place.”
To clarify the factors that govern circuit formation, Dr. Wallace’s team studied how light-sensitive cells called photoreceptors migrate and connect with other cells during development. The team was particularly interested in rod photoreceptors—a type of neuron that responds to dim light.
The researchers used techniques that label individual cells to track the location and behaviour of rods in response to changes in the activity of genes that are involved in cell development and migration.
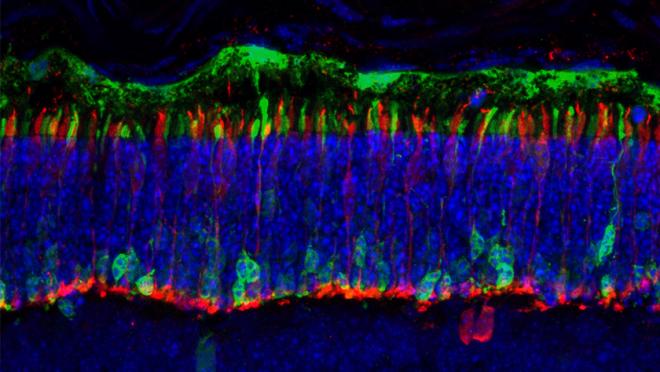
Looking at the photoreceptor layer of the retina under a microscope is like looking at a dense forest where it is difficult to pick out individual trees. The team used fluorescent markers to tag and study the location and behaviour of individual rods. Pictured: a microscopy image of the retina with tagged rod photoreceptors shown in green.
“When we started this project, we likened the photoreceptor layer to a cobblestone street—where stones can be added from any direction,” says Dr. Akshay Gurdita, a postdoctoral researcher in Dr. Wallace's lab and the lead author of the study. “But we learned that this layer is more like a brick wall, with younger photoreceptors added on top of older ones.”
The team also discovered that all rods start at the top of the layer and move downwards over time.
This movement is guided by two factors. Initially, younger cells push older cells downwards as they jostle for space. Then, each cell moves to its final position as it matures and connects with other cells. If either of these processes is disrupted, photoreceptors fail to position properly.
“Our findings highlight the importance of two factors for determining rod positioning: those that are inherent to the cell itself—namely, maturation and the process of wiring with other cells—and those that are external to the cell—namely the proliferation of new cells and resulting space constraints,” explains Dr. Gurdita.
The team’s findings have implications for developing cell-based therapies to treat neuron loss and restore vision.
Several research teams, including Dr. Wallace’s group, are exploring photoreceptor transplantation as a treatment for retinal degeneration. Unfortunately, progress in this area has been limited by transplanted cells failing to integrate into the recipient retina.
“Our findings are very exciting because they point us to processes that we can target to coax donor cells to form functional connections with host cells,” says Dr. Wallace. “If we transplant more mature cells that are ready to form connections or modify the cells and chemical signals that are present in the host retina, we might be able to improve cell integration and restore vision.”
“Eventually, we might also be able to measure donor cell maturation and connectivity to predict the success of a cell transplantation before vision changes occur,” adds Dr. Gurdita.
This work was supported by the Canada First Research Excellent fund-Medicine by Design University of Toronto, the Natural Sciences and Engineering Research Council, the Government of Ontario, the UofT-UHN Vision Science Research Program, the Ontario Institute for Regenerative Medicine, Foundation Fighting Blindness Canada, the Krembil Research Institute, Krembil Foundation and UHN Foundation. Dr. Valerie Wallace is a Professor in the Department of Ophthalmology and Vision Sciences and holds a Tier I Canada Research Chair in Retina Regeneration.
Gurdita A, Pham Truong VQB, Dolati P, Juric M, Tachibana N, Liu ZC, Ortín-Martínez A, Ibrahimi M, Pokrajac NT, Comanita L, Pacal M, Huang M, Sugita S, Bremner R, Wallace VA. Progenitor division and cell autonomous neurosecretion are required for rod photoreceptor sublaminar positioning. Proc Natl Acad Sci U S A. 2023 Oct 17;e2308204120. doi: 10.1073/pnas.2308204120. Epub 2023 Oct 9.

Dr. Akshay Gurdita working in the Wallace Laboratory.




