By: Laura Kuhlmann, Postdoctoral Fellow at Princess Margaret Cancer Centre
The principle of cancer immunotherapy is to leverage a patient’s own immune system to attack cancer cells. Scientists continue to refine this technique since it was first reported nearly 130 years ago. The reduced toxicity of such therapeutic approaches compared to classical chemotherapy, combined with the possibility of eliciting long-lasting effects, tailored to best suit the patient’s needs, makes the field of cancer immunotherapy extremely appealing.
UHN has several talented researchers dedicated to developing novel cancer immunotherapies and offers unique opportunities for trainees to become involved in cutting edge research.
Diverse strategies can be employed to achieve an anti-tumour immune response, including:
- Antibody-based therapies
- Cytokine therapies
- Cellular immunotherapies
Antibody-based therapies use monoclonal antibodies (MAB) which recognize antigens either on the surface of cancer cells or on the surface of immune cells. Dr. Thomas Kislinger, Senior Scientist at the Princess Margaret Cancer Centre specializes in analyzing the cell-surface proteome, which is often underrepresented in proteomic datasets due to the poor solubility and reduced abundance of plasma membrane proteins. Dr. Kislinger’s team subsequently employs bioinformatic strategies and data mining to identify novel promising therapeutic targets. MABs against potential targets are then raised in collaboration with adMare BioInnovations (formerly CDRD), located in Vancouver.
Alternatively, cytokine-based therapies are employed to enhance existing anti-tumour responses and are most often administered as combination therapies or coupled to MABs. Stimulatory cytokines can also be released by tumor cells in response to radiotherapy. Dr. Shane Harding, Scientist at the Princess Margaret Cancer Centre investigates how radiotherapy can initiate cellular signaling and influence patient responses to immunotherapy.
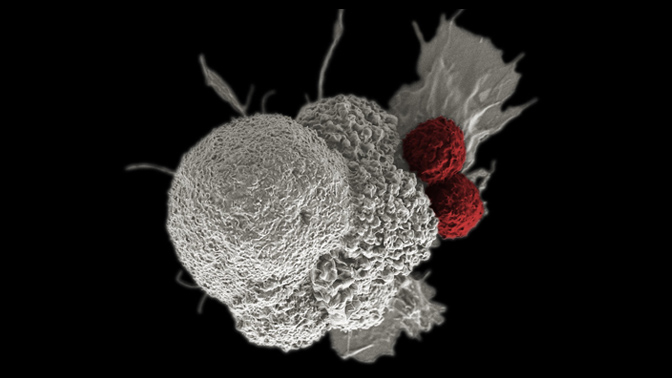
Dr. Marcus Butler, Clinical Head of the Immune Monitoring Team at the Princess Margaret Cancer Centre and Co-leader of the Immuno-oncology Translational Research Initiative, focuses his efforts on making tumors immunologically active using both cellular immunotherapies (adoptive T-cell therapy) and checkpoint blockade approaches. In adoptive T-cell therapies a patient’s T-cells are isolated, a tumour-reactive subset is expanded and subsequently reinfused in the patient. Dr. Butler’s team has been involved in clinical trials evaluating the feasibility of combination therapies to enhance tumour-reactive T-cell survival in vivo, thus establishing anti-tumour immunologic memory. However, the tumour microenvironment is highly immunosuppressive. The Immuno-oncology Translational Research Initiative also aims to identify those patients who respond best to immunotherapies by studying in-depth their genome and immune system. Ultimately, Dr. Butler’s approaches open the door for personalized treatment in oncology and can offer long-lasting therapeutic effects derived from immunologic memory.
Therefore, by stimulating the patient’s own immune system to identify and eliminate genetically heterogeneous cancer cells, oncologic immunotherapies offer less-toxic and potentially personalized treatments against constantly evolving tumours. Collaborations within the institute and with industry partners will be necessary to translate leading research into new clinical therapies.

