
October 2012
Welcome to Net Results EXPRESS
Net Results EXPRESS (NRx) is an award-winning, monthly e-newsletter highlighting medical and scientific breakthroughs, major grants and honours awarded and other research-related events at UHN. Through NRx you can read about ongoing research at our five research institutes, the Ontario Cancer Institute (OCI), the Toronto General Research Institute (TGRI), the Toronto Western Research Institute (TWRI), the Toronto Rehabilitation Institute (TRI) and the Techna Institute for the Advancement of Technology for Health (TECHNA). We hope you will find this newsletter informative and helpful. If you have feedback or questions, please contact www@uhnresearch.ca. Christopher J. Paige, PhD, FCAHS |
Cancer: A Single DNA Nucleotide Difference Regulates Breast Cancer Cell Growth
Dr. Lupien’s study, which was reported in a recent Nature Genetics paper, used a novel computational method known as variant set enrichment (VSE) to show that breast cancer risk-associated SNPs preferentially target the elements that regulate gene expression instead of directly targeting genes to promote cancer growth as previously thought. Using a second novel computational method termed IntraGenomics Replicate, Dr. Lupien’s group showed that risk-associated SNPs commonly alter the affinity of the chromatin for proteins that regulate gene expression. Focusing on a specific risk-associated SNP, this study demonstrated that a single nucleotide change is sufficient to increase the affinity of chromatin for a protein known as forkhead box protein A1. This resulted in decreased expression of TOX-3, a protein known to repress cell growth. The presence of this breast cancer risk-associated SNP would therefore promote uncontrolled cancer cell growth. Altogether there are more than 4800 genetic risk-associated SNPs with more than 500 human traits and disorders. As Dr. Lupien explains, “The methods used in this study provide a mechanism by which we can prioritize the overabundance of putative SNPs and advance the characterization of risk variants.” Breast cancer risk-associated SNPs modulate the affinity of chromatin for FOXA1 and alter gene expression. Cowper-Sal Lari R, Zhang X, Wright JB, Bailey SD, Cole MD, Eeckhoute J, Moore JH, Lupien M. Nature Genetics. 2012 September 23. [Pubmed abstract] This work was supported by the US National Cancer Institute, the American Cancer Society, the US National Institutes of Health and The Princess Margaret Hospital Foundation. |
Diabetes: The Role of the Brain in Insulin Resistance
Current knowledge of the brain’s response to insulin is centered on the role of the hypothalamus, which acts to shutdown the body’s production of sugar. Recent findings by TGRI Scientist Dr. Tony Lam have identified another region of the brain—known as the dorsal vagal complex (DVC)—as important for this process. Using an experimental model, Dr. Lam’s group compared the insulin response of the hypothalamus with that of the DVC and found that, similarly to the hypothalamus, the DVC responded to insulin by reducing sugar production; however, the DVC did so through a different cellular pathway. While the hypothalamus acted via PI3-Kinase (PI3K) signalling, the DVC acted by way of an alternate MAP-Kinase (MAPK-1/2) pathway. Furthermore, experimental models showed that high-fat diets were able to restrict the action of the hypothalamus and the DVC in response to insulin, implicating both in insulin resistance. Dr. Lam emphasises that by discovering a new insulin-responsive pathway within the brain, “Our grasp of the cellular mechanisms of insulin resistance have been greatly improved, with important implications for the development of new treatments for type 2 diabetes.” Insulin activates erk1/2 signaling in the dorsal vagal complex to inhibit glucose production. Filippi BM, Yang CS, Tang C, Lam TK. Cell Metabolism. 2012 October 3. [Pubmed abstract] This work was supported by the Canadian Diabetes Association, the Ontario Ministry of Economic Development and Innovation and the John Kitson McIvor Endowed Chair in Diabetes Research. Dr. Lam is a Tier 2 Canada Research Chair in Obesity Research. |
Malaria: Blood Type Influences Disease Severity
Studying blood cells from subjects with the various blood types, TGRI Senior Scientist Dr. Kevin Kain and collaborators were able to demonstrate that the immune system was more effective at clearing out infected group O red blood cells. Macrophages—immune cells responsible for the removal of infectious agents—destroyed malaria-infected O cells more avidly than infected A or B cells. By using a technique to remove the part of the membrane factor that identifies a blood cell as A or B—functionally transforming them all to act like group O cells—the researchers were able to show that the findings were indeed due to the blood group molecules. The protective effect of group O is observed only after infection, as the malaria parasite invaded and matured similarly in all blood types tested. Commenting on the findings, Dr. Kain says “The enhanced clearance of infected O blood cells may represent a novel mechanism by which blood group O protects against severe malaria.” Wolofsky KT, Ayi K, Branch DR, Hult AK, Olsson ML, Liles WC, Cserti-Gazdewich CM, Kain KC. ABO blood groups influence macrophage-mediated phagocytosis of Plasmodium falciparum-infected erythrocytes. PLoS Pathogens. 2012 October. [Pubmed abstract] This work was supported by the Canadian Institutes of Health Research, the Swedish Research Council, the Lund University Hospital, Region Skane and Mr. Kim Kirkland. Dr. Kain is a Tier 1 Canada Research Chair in Molecular Parasitology. |
Stroke: An Experimental Model Accurately Predicts the Outcomes of a Therapeutic Stroke Drug
TWRI Senior Scientist Dr. Michael Tymianski has developed a new experimental model that may help address this problem. Dr. Tymianski and his team designed a controlled experimental model that reproduces the conditions used in a human clinical trial, known as the ENACT (Evaluating Neuroprotection in Aneurysm Coiling Therapy) trial. ENACT was conducted to evaluate the ability of a new drug—Tat-NR2B9c—to reduce damage induced by small strokes in patients undergoing surgery. When the team independently tested the effects of Tat-NR2B9c in their model and compared them to the results seen in the ENACT trial, they found that the neuroprotectant effects of Tat-NR2B9c were similar; both decreased the number of strokes by about 50-60%. This is the first experimental model to accurately predict the outcomes of a corresponding human clinical trial and could therefore be useful when evaluating novel neuroprotectants prior to their use in humans. A translational paradigm for the preclinical evaluation of the stroke neuroprotectant Tat-NR2B9c in gyrencephalic nonhuman primates. Cook DJ, Teves L, and Tymianski M. Science Translational Medicine. 2012 October 3. [Pubmed abstract] This work was supported by the Canadian Stroke Network. Dr. Tymianski is a Tier 1 Canada Research Chair in Translational Stroke Research. |
Genetics: Protein Levels Associated with Behavioural Impairments
Rett Syndrome is caused by mutations in the gene that is responsible for making a protein called Methyl-CpG Binding Protein 2 (MeCP2). Dr. Eubanks used an experimental model to observe if any relationships exist between MeCP2 deficiency in different parts of the brain and various observed behaviour. MeCP2 protein levels were found to vary significantly across different parts of the brain, and its levels in specific regions predicted the severity of specific symptoms. In particular, reduced cortical protein levels were linked to general severity, while protein levels in the hippocampus were associated with elevated anxiety-like behaviour. A better understanding of how the amounts of MeCP2 protein govern certain behaviour patterns is a key first step to providing a treatment for this condition. Dr. Eubanks explains, “Partial restoration of MeCP2 to specific regions of the brain may improve behaviour associated with the compromised function of that region.” Regional MeCP2 expression levels in the female MeCP2-deficient mouse brain correlate with specific behavioral impairments. Wither RG, Lang M, Zhang L, Eubanks JH. Experimental Neurology. 2012 September 26. [Pubmed abstract] This work was supported by the the Canadian Institutes of Health Research and the Ontario Rett Syndrome Association. |
Heart Disease: Timing of Depression on Patient Outcomes
This meta-analysis confirmed that CHD patients with depression, developed before or after CHD diagnosis, were 1.75 times more likely to experience a worsening of cardiac health or die of CHD. Specifically, these symptoms were 1.79 times more likely to occur in patients suffering from depression before CHD diagnosis, 2.11 times more likely in patients suffering from depression after CHD diagnosis, and 1.59 times more likely in patients who suffered from depression before and after CHD diagnosis. “Development of depression at any time around the point a patient is diagnosed with CHD poses further complications to their health,” says Dr. Grace. “There are proven therapies that improve mood in these patients, although few patients gain access to them. Moreover, we need to further investigate what type of therapeutic interventions can improve their prognosis.” The impact of premorbid and postmorbid depression onset on mortality and cardiac morbidity among patients with coronary heart disease: meta-analysis. Leung YW, Flora DB, Gravely S, Irvine J, Carney RM, Grace SL. Psychosomatic Medicine. 2012 October. [Pubmed abstract] This work was supported by the the Canadian Institutes of Health Research. |
 |
![]()
 Congratulations to TGRI Senior Scientist Dr. Kevin Kain, who received a 4 year, $1.25M award from the Preventing Preterm Birth (PPB) initiative—a Grand Challenge in Global Health administered by the Global Alliance to Prevent Prematurity and Stillbirth (GAPPS), an initiative of Seattle Children’s. The program seeks to discover biological mechanisms that lead to preterm birth and develop novel interventions to prevent them.
Congratulations to TGRI Senior Scientist Dr. Kevin Kain, who received a 4 year, $1.25M award from the Preventing Preterm Birth (PPB) initiative—a Grand Challenge in Global Health administered by the Global Alliance to Prevent Prematurity and Stillbirth (GAPPS), an initiative of Seattle Children’s. The program seeks to discover biological mechanisms that lead to preterm birth and develop novel interventions to prevent them.Dr. Kain is the current Director of the SAR Laboratories, Sandra Rotman Centre for Global Health, Director of the Center for Travel and Tropical Medicine at TGH, holds a Tier 1 Canada Research Chair in Molecular Parasitology, and is a Professor of Medicine at the University of Toronto. PPB grant funding will be used towards Dr. Kain’s research into the discovery of new biological signatures, or biomarkers, and interventions to prevent preterm birth and stillbirth associated with placental malaria.
GAPPS leads a collaborative, global effort to increase awareness and accelerate innovative research and interventions that will improve maternal, newborn and child health outcomes around the world. To establish the PPB initiative, a grant was provided to GAPPS from The Bill and Melinda Gates Foundation.
 Dr. Nostro’s research program will focus on the generation of functional pancreatic beta cells from human embryonic and induced pluripotent stem cells via directed differentiation and reprogramming strategies, with the ultimate goal of translating their discoveries to potential treatments for Type I diabetes patients. She completed her PhD at the University of Manchester and a postdoctoral fellowship at the Mount Sinai School of Medicine and UHN. Dr. Nostro also holds the Harry Rosen Chair in Diabetes and Regenerative Medicine Research at the McEwen Centre for Regenerative Medicine.
Dr. Nostro’s research program will focus on the generation of functional pancreatic beta cells from human embryonic and induced pluripotent stem cells via directed differentiation and reprogramming strategies, with the ultimate goal of translating their discoveries to potential treatments for Type I diabetes patients. She completed her PhD at the University of Manchester and a postdoctoral fellowship at the Mount Sinai School of Medicine and UHN. Dr. Nostro also holds the Harry Rosen Chair in Diabetes and Regenerative Medicine Research at the McEwen Centre for Regenerative Medicine. Dr. Haycock will be working on the real-time systems of the Challenging Environments Assessment Lab, including the advanced driving simulator currently under development. He completed his PhD in Aerospace Engineering at the University of Toronto Institute for Aerospace Studies, where his research involved improving real-time helicopter dynamics modelling.
Dr. Haycock will be working on the real-time systems of the Challenging Environments Assessment Lab, including the advanced driving simulator currently under development. He completed his PhD in Aerospace Engineering at the University of Toronto Institute for Aerospace Studies, where his research involved improving real-time helicopter dynamics modelling.Feedback
Net Results EXPRESS is brought to you by UHN Research Communications. We hope you have enjoyed receiving this message. If you have any feedback, please email www@uhnresearch.ca.
To access archived issues of Net Results EXPRESS, visit uhnresearch.ca/news/netresultsexpress
Some images adapted from the image archives of stock.xchng.ca and Wikipedia. OCI Scientist Dr.
OCI Scientist Dr. 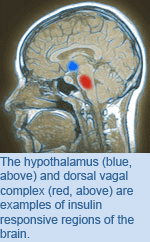 Insulin resistance is responsible for almost 90% of all cases of diabetes. High-fat diets and obesity have been linked to this condition, in which insulin released into the blood stream shows no effect on target organs. Normally these organs respond to insulin by reducing sugar production or increasing sugar uptake from the blood.
Insulin resistance is responsible for almost 90% of all cases of diabetes. High-fat diets and obesity have been linked to this condition, in which insulin released into the blood stream shows no effect on target organs. Normally these organs respond to insulin by reducing sugar production or increasing sugar uptake from the blood.  Blood groups are determined by the type of molecules present on the surface of red blood cells. In the ABO blood group system, a cell can express the A type, B type, both (leading to the AB blood type) or neither (leading to the O blood type). Group O predominates in populations with a long history of coexisting with malaria, and studies suggest that malaria is less severe in those with group O blood than those with groups A or B.
Blood groups are determined by the type of molecules present on the surface of red blood cells. In the ABO blood group system, a cell can express the A type, B type, both (leading to the AB blood type) or neither (leading to the O blood type). Group O predominates in populations with a long history of coexisting with malaria, and studies suggest that malaria is less severe in those with group O blood than those with groups A or B. During a stroke, blood stops flowing through regions of the brain, which results in neurological damage. One of the proposed strategies for treating stroke is the use of neuroprotectants—drugs that protect the brain from the damage inflicted during the stroke. Experimental studies in animals such as mice and rats indicate that some neuroprotectants could be effective at reducing the neurological damage induced by strokes; however none of these drugs have been translated into effective stroke therapies for humans. There are a number of reasons that could explain this, one of which might be the fact that the stringent conditions used in experimental studies fail to fully replicate the conditions that occur in real life.
During a stroke, blood stops flowing through regions of the brain, which results in neurological damage. One of the proposed strategies for treating stroke is the use of neuroprotectants—drugs that protect the brain from the damage inflicted during the stroke. Experimental studies in animals such as mice and rats indicate that some neuroprotectants could be effective at reducing the neurological damage induced by strokes; however none of these drugs have been translated into effective stroke therapies for humans. There are a number of reasons that could explain this, one of which might be the fact that the stringent conditions used in experimental studies fail to fully replicate the conditions that occur in real life. Rett Syndrome is a developmental disorder that impacts the brain and primarily affects females. Symptoms develop around 6–18 months of age and include the loss of speech, hand skills and gait, as well as impaired social interactions. However, little is understood as to why symptom severity differs so vastly between those afflicted. TWRI Senior Scientist Dr.
Rett Syndrome is a developmental disorder that impacts the brain and primarily affects females. Symptoms develop around 6–18 months of age and include the loss of speech, hand skills and gait, as well as impaired social interactions. However, little is understood as to why symptom severity differs so vastly between those afflicted. TWRI Senior Scientist Dr.  Depression is associated with an increased risk in developing coronary heart disease (CHD) and an increased risk in death from CHD. Worryingly, 20% of patients diagnosed with CHD are also subsequently diagnosed with depression, further aggravating their condition. Recently, a number of studies have examined whether the time frame of depression development (before or after CHD diagnosis) affects the health of a CHD patient; however, inconsistent findings have been reported. To address these differences, TRI Adjunct Scientist, TGRI Scientist and York University Associate Professor Dr.
Depression is associated with an increased risk in developing coronary heart disease (CHD) and an increased risk in death from CHD. Worryingly, 20% of patients diagnosed with CHD are also subsequently diagnosed with depression, further aggravating their condition. Recently, a number of studies have examined whether the time frame of depression development (before or after CHD diagnosis) affects the health of a CHD patient; however, inconsistent findings have been reported. To address these differences, TRI Adjunct Scientist, TGRI Scientist and York University Associate Professor Dr.