 Welcome to the March issue of NRx (formerly known as Net Results Express). Through NRx you can read about ongoing research at our five research institutes, the Ontario Cancer Institute (OCI), the Toronto General Research Institute (TGRI), the Toronto Western Research Institute (TWRI), the Toronto Rehabilitation Institute (TRI) and the Techna Institute (Techna).
Welcome to the March issue of NRx (formerly known as Net Results Express). Through NRx you can read about ongoing research at our five research institutes, the Ontario Cancer Institute (OCI), the Toronto General Research Institute (TGRI), the Toronto Western Research Institute (TWRI), the Toronto Rehabilitation Institute (TRI) and the Techna Institute (Techna).
In this month’s issue you can read about research in:
- The use of deep brain stimulation in the treatment of anorexia
- The causes of tumours of the eye
- Targeting hypoxia in cancer therapy
- Glucagon secretion in response to changes in blood sugar levels
- The effectiveness of different referral strategies for women undergoing cardiac rehabilitation
We hope that you will find NRx informative and helpful. If you have feedback or questions, please contact www@uhnresearch.ca.
Christopher J. Paige, PhD, FCAHS
Vice President, Research
University Health Network
Some treated patients completed an inpatient eating disorders program for the first time.
In a world first, a team of UHN scientists, led by TWRI Senior Scientist Dr. Andres Lozano, have shown that deep brain stimulation (DBS) in patients with anorexia helps to achieve and maintain improvements in body weight, mood and anxiety. DBS is a surgical procedure in which electrodes are implanted into specific parts of the brain to moderate the activity of dysfunctional areas. Anorexia is an eating disorder and psychiatric condition characterized by food restriction, body distortion and an overwhelming fear of gaining weight.
After a nine-month period following DBS surgery, the team observed that the patients had achieved weight gain—for these patients it was the longest period of weight gain since the onset of their illness. Patients also exhibited simultaneous changes in mood, anxiety, control over emotional responses, urges to binge and purge and other symptoms related to anorexia. “We are truly ushering in a new of era of understanding of the brain and the role it can play in certain neurological disorders,” says Dr. Lozano. “By pinpointing and correcting the precise circuits in the brain associated with the symptoms of some of these conditions, we are finding additional options to treat these illnesses.”
This work was supported by the Klarman Family Foundation and the Canadian Institutes of Health Research. A. Lozano is a Tier 1 Canada Research Chair in Neuroscience.
Subcallosal cingulate deep brain stimulation for treatment-refractory anorexia nervosa: a phase 1 pilot trial. Lipsman N, Woodside DB, Giacobbe P, Hamani C, Carter JC, Norwood SJ, Sutandar K, Staab R, Elias G, Lyman CH, Smith GS, Lozano AM. Lancet. 2013 March 6. [Pubmed abstract]
Oncogene-driven tumours are generally larger than the inherited variety.
Retinoblastoma, a cancer of the eye, typically develops because of a mutation to the retinoblastoma 1 (RB1) tumour suppressor gene—a gene that normally prevents the formation of cancer—that is inherited from parents. However, retinoblastoma can also occur in patients with no family history of the disease, this is known as non-familial retinoblastoma. In a multi-centre study, led by OCI Senior Scientist Dr. Brenda Gallie, a new type of retinoblastoma was discovered providing a possible explanation as to why non-familial retinoblastomas occur.
The researchers analysed more than 1000 non-familial retinoblastoma tumours and found that a single oncogene—a cancer-promoting gene—drove tumour growth . “When we remove the eye with a large tumour and show that it is the new oncogene-driven type of retinoblastoma, there is believed to be zero risk for retinoblastoma developing in the other eye or in other members of the family. This is a major advance in personalized cancer medicine for these patients," says Dr. Gallie.
This work was supported by the National Institutes of Health, the Canadian Institutes of Health Research, the Canadian Retinoblastoma Society, Hyland Foundation, Toronto Netralaya and Doctors Lions Club, the Alcon Research Institute, the Ontario Ministry of Health and Long-Term Care and The Princess Margaret Cancer Foundation.
Characterisation of retinoblastomas without RB1 mutations: genomic, gene expression, and clinical studies. Rushlow DE, Mol BM, Kennett JY, Yee S, Pajovic S, Thériault BL, Prigoda-Lee NL, Spencer C, Dimaras H, Corson TW, Pang R, Massey C, Godbout R, Jiang Z, Zacksenhaus E, Paton K, Moll AC, Houdayer C, Raizis A, Halliday W, Lam WL, Boutros PC, Lohmann D, Dorsman JC, Gallie BL. Lancet Oncology. 2013 March 12. [Pubmed abstract]
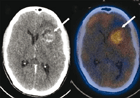
Medical imaging can detect hypoxic regions in tumours.
A lack of oxygen, known as hypoxia, is a common condition for cancer cells in solid tumours. Hypoxia in tumours is a major limiting factor in successful cancer treatment; hypoxic cells require nearly three times as much radiation to kill. OCI Senior Scientist Dr. Bradly Wouters and collaborators studied the mechanisms that confer hypoxia resistance to cancer cells and recently identified one signalling pathway that, when inhibited, could improve the response to treatment.
The hypoxia inducible factor pathway (HIF) and protein kinase r-like endoplasmic reticulum kinase (PERK)/eukaryotic initiation factor 2 α (eIF2α) both play a role in helping cancer cells survive the hypoxic environment of a tumour. Inhibiting either pathway decreased the fraction of surviving cells in hypoxic zones to a similar extent. HIF inhibition did not result in an improved response to radiation therapy, whereas blocking the PERK/eIF2α pathway led to “a striking improvement in tumour response to treatment,” according to Dr. Wouters. The study demonstrated that the PERK/eIF2α pathway mediates resistance to repeated cycles of hypoxia and oxygenation in tumours and that these cells were particularly important for the recovery and growth of tumours following radiation therapy.
This work was supported by the Dutch Science Organization, the Dutch Cancer Society, the Ontario Ministry of Health and Long-Term Care, The Terry Fox Research Institute, The Ontario Institute for Cancer Research, The Canadian Institutes of Health Research, The European Union Seventh Framework Programme/METOXIA and The Princess Margaret Cancer Foundation.
PERK/eIF2α signaling protects therapy resistant hypoxic cells through induction of glutathione synthesis and protection against ROS. Rouschop KM, Dubois LJ, Keulers TG, van den Beucken T, Lambin P, Bussink J, van der Kogel AJ, Koritzinsky M, Wouters BG. Proceedings of the National Academy of Sciences. 2013 March 19. [Pubmed abstract]
α-cells in the pancreas are responsible for synthesizing and secreting glucagon when blood glucose levels fall too low.
Glucagon—a hormone secreted by pancreatic islet α-cells when blood sugar (glucose) levels fall too low—is important for maintaining normal blood glucose levels during fasting and starvation. While it is known that glucagon secretion is abnormal in in type 1 and 2 diabetics, why this occurs is not understood. A recent study from TGRI Senior Scientist Dr. Michael Wheeler and postdoctoral fellow Dr. Emma Allister sheds light on this mystery by showing that UCP2, a protein expressed in many tissues including islet cells, plays a key role in regulating glucagon secretion.
The team created an experimental model in which the UCP2 gene is deleted specifically from the α-cells of the pancreas. The model showed reduced levels of glucagon during fasting and impaired glucagon secretion when compared to controls. Moreover, these effects could be mimicked by applying a drug that blocks the activity of UCP2 on mouse and human islet cell cultures. “These results suggest that increased UCP2 activity is needed to promote glucagon secretion,” states Dr. Wheeler. “Conversely, increased activity or expression of UCP2 may also contribute to the abnormal glucagon secretion and elevated blood sugar that is observed in diabetes.”
This work was supported by the Canadian Institutes of Health Research.
UCP2 Regulates the Glucagon Response to Fasting and Starvation. Allister EM, Robson-Doucette CA, Prentice KJ, Hardy AB, Sultan S, Gaisano HY, Kong D, Gilon P, Herrera PL, Lowell BB and Wheeler MB. Diabetes. 2013 February 22. [Pubmed abstract]

CR programs include exercise training, education and counselling.
Heart disease is the leading cause of death in women around the world. Cardiac rehabilitation (CR) is known to reduce death rates among these patients and improve their quality of life. Despite these benefits, women are less likely to be referred to CR than men. To better understand why this happens, a multi-centre study, led by TRI Adjunct Scientist and TGRI Scientist Dr. Sherry Grace, examined the different strategies used by Ontario hospitals to refer women to CR.
Cardiovascular disease patients completed a survey that assessed their CR referral and usage. Referral strategies used by hospitals included an automatic referral after discharge, a referral after a personal discussion with a health care provider, a combination of the two strategies or the current method of referral at the discretion of the health care provider. “We found that the combination of automatic and personal discussion-based referral strategies resulted in the greatest CR enrolment rates among women—10 times greater than the current method of referral,” says Dr. Grace. “Health care providers should be encouraged to discuss CR with all eligible women patients.”
This work was supported by the Canadian Institutes of Health Research and Heart and Stroke Foundation of Canada.
Effect of referral strategies on access to cardiac rehabilitation among women. Gravely S, Anand SS, Stewart DE, Grace SL; on behalf of the CRCARE Investigators. European Journal of Preventive Cardiology. 2013 March 11. [Pubmed abstract]
 UHN welcomes Dr. Kara Patterson, TRI Scientist and Assistant Professor in the Department of Physical Therapy at the University of Toronto. Dr. Patterson joins the Mobility Team at TRI. Her research interests include the neural control of gait and how it is affected post stroke, motor re-learning of lower extremity movements post stroke and measurement and neurorehabilitation of gait.
UHN welcomes Dr. Kara Patterson, TRI Scientist and Assistant Professor in the Department of Physical Therapy at the University of Toronto. Dr. Patterson joins the Mobility Team at TRI. Her research interests include the neural control of gait and how it is affected post stroke, motor re-learning of lower extremity movements post stroke and measurement and neurorehabilitation of gait.