 As UHN's monthly research newsletter, NRx reports on the full spectrum of research from UHN's five research institutes: the Princess Margaret (PM) Cancer Centre, the Toronto General Research Institute (TGRI), the Toronto Western Research Institute (TWRI), the Toronto Rehabilitation Institute (TRI) and the Techna Institute (Techna).
As UHN's monthly research newsletter, NRx reports on the full spectrum of research from UHN's five research institutes: the Princess Margaret (PM) Cancer Centre, the Toronto General Research Institute (TGRI), the Toronto Western Research Institute (TWRI), the Toronto Rehabilitation Institute (TRI) and the Techna Institute (Techna).
In this issue you can read about:
- An effective new treatment for obsessive compulsive disorder
- The identification of a protein that is critical for heart function
- New insight into what Alzheimer plaques are made of
- How insomnia may limit recovery after concussions
- How the eye connects to the visual regions of the brain
- A health care gap that limits cancer treatment in low-income countries
We hope that you will find NRx informative. If you have feedback or questions, please contact www@uhnresearch.ca.
Christopher J. Paige, PhD, FCAHS
Executive Vice President, Science and Research
University Health Network
New image guided therapy shows promise for those with hard-to-treat obsessive compulsive disorder

Unbalanced connectivity between brain regions is associated with behavioural disorders such as OCD, anxiety and depression.
Obsessive compulsive disorder (OCD) is a devastating illness characterized by intrusive and persistent thoughts (obsessions) as well as disruptive and repetitive behaviours (compulsions). People afflicted with OCD are unable to control their obsessions or compulsions and close to 60% of them do not respond to traditional drug therapy.
Communication errors between certain brain regions (known as abnormal connectivity) is thought to underlie OCD. Thus, therapies aimed at restoring normal brain connectivity offer a novel approach to OCD treatment. Preliminary trials using surgically based therapies, such as deep brain stimulation, have shown promise but there is a need for more accessible and non-invasive alternatives.
TWRI Scientist Dr. Jonathan Downar and collaborators devised a new approach to treat OCD. First, they used repeated transcranial magnetic stimulation (rTMS) to restore normal connectivity between affected brain regions. The application of rTMS is a painless and non-invasive approach that involves placing a magnetic field on patients' scalps to influence electrical activity in the brain. Second, they used an imaging technique to confirm the regions of OCD patients' brains that displayed abnormal connectivity before treatment and how that connectivity changed after treatment.
Dr. Downar comments, "Of those treated, half responded favourably to rTMS—a remarkable finding considering that all of these patients were non-responsive to traditional therapy. The next step will be to refine our brain imaging method to better customize the rTMS treatment for each individual patient, with the aim of further improving treatment response."
This work was supported by the Canadian Institutes of Health Research, the National Institutes of Health, the Klarman Family Foundation, the Buchan Family Foundation and the Toronto General & Western Hospital Foundation.
Reductions in cortico-striatal hyperconnectivity accompany successful treatment of obsessive-compulsive disorder with dorsomedial prefrontal rTMS. Dunlop K, Woodside B, Olmsted M, Colton P, Giacobbe P, Downar J. Neuropsychopharmacology. 2015 Oct 6. [Pubmed abstract]
Research identifies Tmem65 as a key regulator of human heart cell development and function
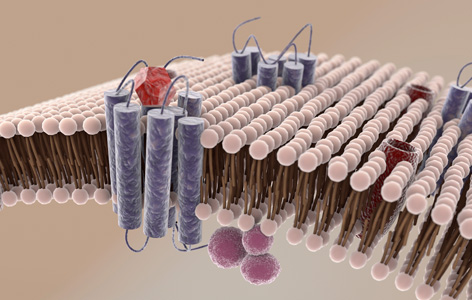
Cell membrane proteins (blue) are difficult to detect because they are often damaged after being extracted from the lipid membrane.
During a heartbeat, electrical signals rapidly travel through heart tissue and instruct each cell to contract. Proteins at regions of the cell membrane known as gap junctions allow the electrical signal to pass between neighbouring cells, leading to concerted contractions.
Although cell membrane proteins are critical in regulating heart function, they have been difficult to study because they are extremely difficult to detect and purify. TGRI Scientist Anthony Gramolini and PM Cancer Centre Senior Scientist Thomas Kislinger have overcome these challenges and identified a set of cell membrane proteins that regulate how gap junctions are formed.
By refining an existing protein purification method, the researchers were able to isolate more than 500 cell membrane proteins from human heart cells. They then analyzed the structures and functions of these isolated proteins and identified a top candidate protein—known as Tmem65—as a possible regulator of human heart development and function. Analysis of Tmem65 function revealed that it regulates another protein, known as Cx43, to form functional gap junctions. Without Tmem65, Cx43 is broken down and electrical signals in the heart are disrupted.
"By identifying a key role for Tmem65 in gap function biology, these findings provide new insight into cardiac diseases such as arrhythmias, which occur when channels that conduct electrical signals fail to function properly, " explain Drs. Kislinger and Gramolini. "We are now actively studying the functions of other proteins that we identified with the aim of uncovering new therapeutic targets for cardiac disease."
This work was supported by the Heart and Stroke Foundation of Ontario, the Canadian Institutes of Health Research, the Heart and Stroke Richard Lewar Centre of Cardiovascular Excellence, the Ontario Research Fund—Global Leadership Round in Genomics and Life Sciences, the Ontario Genomics Institute, the Canada Foundation for Innovation, the Natural Sciences and Engineering Research Council of Canada, the Canadian Cystic Fibrosis Foundation, the Canadian Cancer Society, Novartis, the German Research Council, the German Federal Ministry for Science and Education, the National Institutes of Health, The Princess Margaret Cancer Foundation and the McEwen Centre for Regenerative Medicine. A Gramolini holds a Tier 2 Canada Research Chair (CRC) in Cardiovascular Proteomics and Molecular Therapeutics, T Kislinger holds a Tier 2 CRC in Proteomics in Cancer Research and G Keller holds a Tier 1 CRC in Embryonic Stem Cell Biology.
Evolutionarily conserved intercalated disc protein Tmem65 regulates cardiac conduction and connexin 43 function. Sharma P, Abbasi C, Lazic S, Teng AC, Wang D, Dubois N, Ignatchenko V, Wong V, Liu J, Araki T, Tiburcy M, Ackerley C, Zimmermann WH, Hamilton R, Sun Y, Liu PP, Keller G, Stagljar I, Scott IC, Kislinger T, Gramolini AO. Nature Communications. 2015 Sep 25. [Pubmed abstract]
New approach lets researchers see which proteins make up diseased and healthy tissues
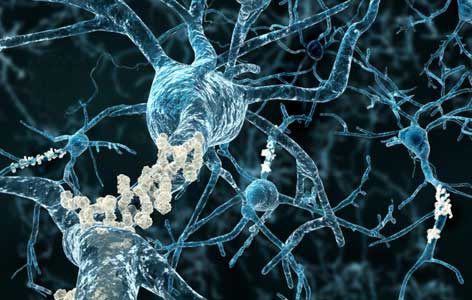
In Alzheimer disease, proteins clump together near neurons (blue) to form harmful amyloid plaques (beige) of varying sizes.
The presence of abnormal clumps of proteins (known as "plaques") within the brain is a defining feature in a number of neurological disorders, including Alzheimer disease. Identifying the proteins present in these plaques provides researchers with important molecular targets that could be used to develop new therapeutic strategies.
While advanced approaches exist that rely on mass spectrometry (MS) and standard imaging techniques to identify proteins, these methods are limited by low resolutions. As such, these models cannot be used to identify proteins located in very small, irregular or complex tissue features.
To overcome these limitations, PM Senior Scientist Dr. Avijit Chakrabartty and collaborators devised a new approach called STOMP (spatially targeted optical microproteomics). The approach combines MS with a type of imaging known as two-photon microscopy to enable very small tissue features to be analysed.
Dr. Chakrabartty's team used STOMP to isolate plaques in samples from patients with advanced Alzheimer disease and found 60 enriched proteins. The approach identified a number of proteins that had not previously been shown to be enriched in plaques. Explains Dr. Chakrabartty "We found a number of presynaptic proteins, which suggests that synapses—the regions where brain cells, or neurons, communicate—may be involved in development of the disease."
This work was supported by The ALS Society of Canada, The Alzheimer Society of Canada, the Canadian Consortium on Neurodegeneration in Aging and The Princess Margaret Cancer Foundation.
Determining composition of micron-scale protein deposits in neurodegenerative disease by spatially targeted optical microproteomics. Hadley KC, Rakhit R, Guo H, Sun Y, Jonkman JE, McLaurin J, Hazrati LN, Emili A, Chakrabartty A. Elife. 2015 Sep 29. [Pubmed abstract]
Insomnia is linked to depression and may impact recovery after mild traumatic brain injury

According to the Canadian Sleep Society, around 10% of Canadians suffer from persistent insomnia.
In Ontario, almost half a million people live with a brain injury. Of these, many have experienced a mild traumatic brain injury (mTBI)—also known as a concussion—that can lead to persistent symptoms, such as anxiety, headaches, depression and fatigue.
Although the symptoms associated with mTBI are known to be worsened by poor sleep, few studies have explored the connection between mTBI and insomnia. To address this, Dr. Tatyana Mollayeva and TRI Senior Scientist Dr. Angela Colantonio led a study to identify whether insomnia occurs more often in those with mTBI.
The study enrolled 94 people who experienced mTBI at work and had symptoms that persisted longer than normal. The findings revealed that as many as 69% of those in the study group experienced insomnia—a value that was higher than previously thought.
Furthermore, the research team analyzed whether medical, social, behavioural and demographic factors were involved. Dr. Mollayeva explains, "While we found that many factors influence insomnia in these patients, depression was most closely linked to insomnia. Our results strongly suggest that sleep assessment should be incorporated into rehabilitation care after mild brain injury. Finding ways to improve sleep in those with mild traumatic brain injury may speed recovery and reduce the chance of depression."
This work was supported by the Canadian Institutes of Health Research (CIHR) and the Toronto Rehab Foundation. A Colantonio is the Saunderson Family Chair in Acquired Brain Injury Research. T Mollayeva was supported by a CIHR Frederick Banting and Charles Best Graduate Scholarship Award.
Insomnia in workers with delayed recovery from mild traumatic brain injury. Mollayeva T, Mollayeva S, Shapiro CM, Cassidy JD, Colantonio A. Sleep Medicine. 2015 Jul 10. [Pubmed abstract]
Findings reveal how nerves in the eye develop and connect with a visual region of the brain

Glaucoma, which affects more than 70 million people worldwide, may progress unnoticed until symptoms like tunnel vision (simulated above) occur.
When human eyes look at their environment they collect pictures that are sent to the brain. Together these pictures form a visual representation of our world. The eyes transmit visual images to the brain through a cable that is composed of nerve fibres called retinal axons. The retinal axons connect the eye to visual areas of the brain, including the optic tectum (OT).
Unfortunately, retinal axons can degenerate in conditions like glaucoma, eventually leading to irreversible blindness. Currently, it is unknown how developing retinal axons connect with the brain, which has limited the creation of new therapies.
Recent findings from TWRI Senior Scientist Dr. Philippe Monnier have addressed this problem by shedding light on how retinal axons are guided during development. The researchers found that a group of peptides, known as Repulsive Guidance Molecule a (RGMa) subtypes, work in combination to help retinal axons target the OT. One of the subtypes, C-RGMa, inhibits deep projections in the optic tectum, while the other, N-RGMa, promotes deeper projections in the OT. A balance of both peptides ensures that retinal axons connect with the appropriate area of the OT.
Remarks Dr. Monnier, "Our work has uncovered the peptides responsible for ensuring that retinal axons integrate into the correct layer of the OT. These insights may help developing therapies aimed at restoring vision in patients with retinal axon damage."
This work was supported by the Canadian Institutes of Health Research, the Vision Science Research Program of the University of Toronto and the Toronto General & Western Hospital Foundation.
Ƴ-secretase and LARG mediate distinct RGMa activities to control appropriate layer targeting within the optic tectum. Banerjee P, Harada H, Tassew NG, Charish J, Goldschneider D, Wallace VA, Sugita S, Mehlen P, Monnier PP . Cell Death and Differentiation. 2015 Aug 21. [Pubmed abstract]
International Commission reveals lack of access to radiotherapy in low-income countries

(L – R) UHN's Drs. David Jaffray and Mary Gospodarowicz are leading the Lancet Oncology's Radiotherapy Commission.
According to the World Health Organization, 70% of the world's cancer-related deaths occur in Africa, Asia and Central and South America. A recent article published in The Lancet Oncology reveals that as many as 90% of the world's low-income populations in these regions lack adequate access to the radiotherapy services required to treat cancer.
The data was compiled by a panel of international experts on The Lancet Oncology's Radiotherapy Commission, which is led by Dr. David Jaffray (Techna Director and UHN's Executive Vice President, Technology and Innovation), Dr. Mary Gospodarowicz (Medical Director, Princess Margaret Cancer Centre) and Dr. Rifat Atun (Professor of Global Health Systems at Harvard University).
The report predicts that by 2035, more than 12 million new cancer patients could benefit from radiotherapy treatment. Yet, worldwide access to radiotherapy is unacceptably low.
The building of radiotherapy capacity will require a large initial investment; however, the report states that the health and economic benefits of investments would be realized in just 10 to 15 years. "To justify the investment, we only need to look at the remarkable progress made in tackling the enormous challenges of HIV/AIDS and malaria," says Dr. Mary Gospodarowicz. "This gives us the hope and confidence that the same success can be achieved with cancer control and radiotherapy."
Expanding global access to radiotherapy. Atun R, Jaffray DA, Barton MB, Bray F, Baumann M, Vikram B, Hanna TP, Knaul FM, Lievens Y, Lui TY, Milosevic M, O'Sullivan B, Rodin DL, Rosenblatt E, Van Dyk J, Yap ML, Zubizarreta E, Gospodarowicz M. Lancet Oncology. 2015 Sep. [Pubmed abstract]
 Three UHN Researchers have been named Fellows of the Canadian Academy of Health Sciences (CAHS)—an honour that is considered one of the highest distinctions for Canadians in the health sciences community.
Three UHN Researchers have been named Fellows of the Canadian Academy of Health Sciences (CAHS)—an honour that is considered one of the highest distinctions for Canadians in the health sciences community.  Techna and UHN's Technology Development and Commercialization office are pleased to announce that UHN has licensed a new technology for the radiation therapy treatment planning process to
Techna and UHN's Technology Development and Commercialization office are pleased to announce that UHN has licensed a new technology for the radiation therapy treatment planning process to  PM Cancer Centre Senior Scientist Dr.
PM Cancer Centre Senior Scientist Dr.