
June 2012
Welcome to Net Results EXPRESS
Net Results EXPRESS (NRx) is an award-winning, monthly e-newsletter highlighting medical and scientific breakthroughs, major grants and honours awarded, and other research-related events at UHN. Through NRx you can read about ongoing research at our five research institutes, the Ontario Cancer Institute (OCI), the Toronto General Research Institute (TGRI), the Toronto Western Research Institute (TWRI), the Toronto Rehabilitation Institute (TRI) and the Techna Institute for the Advancement of Technology for Health (TECHNA). We hope you will find this newsletter informative and helpful. If you have feedback or questions, please contact www@uhnresearch.ca. Christopher J. Paige, PhD, FCAHS |
Colon Cancer: Understanding Self-Renewal and Resistance
By examining CC-ICs purified from tumours taken from colon cancer patients, the study identified two proteins, ID1 and ID3, that function together to maintain elevated levels of the protein p21, a well-studied protein that is known to protect cells from damage. When CC-ICs were depleted of ID1 and ID3, p21 levels dropped and their ability to self-renew and generate new tumours was decreased. Interestingly, depletion of these two proteins also caused CC-ICs to become more sensitive to oxaliplatin—a chemotherapeutic drug commonly used to treat colorectal cancer. While further investigations into the linkage between CC-IC and chemoresistance are required, new therapeutics that target these self-renewal mechanisms could eradicate these cells. Commenting on potential uses of these new therapies, Dr. O’Brien says, “Combining CC-IC therapies with conventional cancer therapies could increase beneficial effects and lower the likelihood of a recurring resistant tumour.” ID1 and ID3 regulate the self-renewal capacity of human colon cancer-initiating cells through p21. O'Brien CA, Kreso A, Ryan P, Hermans KG, Gibson L, Wang Y, Tsatsanis A, Gallinger S, Dick JE. Cancer Cell. 2012 June 12. [Pubmed abstract] This work was supported by Genome Canada through the Ontario Genomics Institute, the Canadian Institutes of Health Research, the Krandel Fund at Princess Margaret Hospital, the Ontario Ministry of Health and Long-Term Care, the Princess Margaret Hospital Foundation, the Ontario Institute for Cancer Research and a Premier's Summit Award with funds from the Province of Ontario. Dr. Dick is a Tier I Canada Research Chair in Stem Cell Biology. |
Patient Care: A Better Predictor of Heart Failure in Emergency Room Care
In a multicentre study of 86 Ontario hospitals, the medical charts of 12,591 emergency care patients admitted for symptoms of heart failure were examined. A number of health indicators were compared to mortality seven days after initial assessment and these results were used to create the Emergency Care Heart Failure Mortality Risk Grade (EHMRG). Using this system, the team found that predictors of mortality include lower initial systolic blood pressure, oxygen saturation and hemoglobin concentration in the blood. Additional indicators of increased mortality risk include higher leukocyte count, elevated potassium and creatinine levels, and transportation by emergency medical services. EHMRG will help to identify patients most in need while assisting physicians under difficult circumstances. Dr. Lee explains, “The clinician should synthesize the EHMRG with clinical judgment in assessing the appropriate setting for the care of patients with an acute episode of heart failure.” Prediction of heart failure mortality in emergent care. Lee DS, Stitt A, Austin PC, Stukel TA, Schull MJ, Chong A, Newton GE, Lee JS, Tu JV. Annals of Internal Medicine. 2012 June 5. [Pubmed abstract] This work was supported by the Ontario Ministry of Health and Long-Term Care, the Canadian Institutes of Health Research, the Heart and Stroke Foundation of Ontario. Dr. Tu is a Tier I Canada Research Chair in Health Services Research. |
Prostate Cancer: Seeking Out Genetic Variations that Increase Cancer Risk
Focusing on a prostate cancer associated genetic loci, Dr. Lupien and his team adapted this approach to demonstrate that the disease associated risk could be accounted for by a DNA segment physically interacting with the prostate cancer oncogene SOX9 through a chromatin loop to enhance its expression. Dr. Lupien’s group demonstrated that two genetic variations within this segment impact the expression of their target oncogene. Interestingly, one of the variations identified accounts for the prostate cancer risk in European descendants while another is unique to Colombian and African populations. Genome wide studies like this make it easier to determine the function of genetic variations associated with diseases, including cancer, by integrating chromatin and epigenetic cues in identifying the genes they regulate and the different human populations they account for. Understanding these functions will allow researchers to develop innovative therapeutics that integrate a more personalized approach to medicine. Integrative functional genomics identifies an enhancer looping to the SOX9 gene disrupted by the 17q24.3 prostate cancer risk locus. Zhang X, Cowper-Sal Lari R, Bailey SD, Moore JH, Lupien M. Genome Research. 2012 June 4. [Pubmed abstract] This work was supported by the National Institutes of Health, the National Library of Medicine and The Princess Margaret Hospital Foundation. |
Pancreatitis: How Alcohol Damages the Pancreas
Pretreating pancreatic cells with alcohol metabolites was found to cause defective calcium release and a decreased ability to secrete digestive enzymes critical for the breakdown of food. In addition, specialized cells called acinar cells redirected the secretion of digestive enzymes from the normal top end to the bottom end of the cell which decreased the overall efficiency of enzyme secretion; and wherein digestive enzyme activation at the bottom end results in cell destruction and consequently alcoholic pancreatitis. Dr. Gaisano’s study provides more clarity on how alcohol consumption contributes to the development of pancreatitis. These findings will be critical for the development of therapeutics to help patients with pancreatic injury. Dolai S, Liang T, Lam PP, Fernandez NA, Chidambaram S, Gaisano HY. Effects of ethanol metabolites on exocytosis of pancreatic acinar cells in rats. Gastroenterology. 2012 June 15. [Pubmed abstract] This work was supported by the U.S. Army Medical Research Acquisition Activity and the Canadian Institutes of Health Research. |
Health Outcomes: Chronic Joint Pain in the Canadian Population
A significant portion (over 10%) of Canadians reported having chronic joint pain—defined as pain or stiffness on most days for at least one month—without an accompanying diagnosis of arthritis. The people indicating they had some kind of chronic joint pain were overwhelmingly younger, with 87% under the age of 65. These symptoms caused similar impacts on health outcomes as arthritis, including limiting daily activities and the increased usage of health care resources. From a population survey of this nature, researchers are unable to say precisely what the large group of chronic joint symptoms represents. It could be the early, undiagnosed stages of arthritis or other causes of joint pain, such as soft-tissue disorders. Focusing on the public health policy implications, Dr. Badley says, “Whether people reporting chronic joint pain have arthritis or not, our study suggests a need for interventions and advice to manage these symptoms in order to improve the quality of life and reduce the impact on the health care system.” Comparison of health-related outcomes for arthritis, chronic joint symptoms, and sporadic joint symptoms: a population-based study. Canizares M, Badley EM. Arthritis Care & Research. 2012 June 6. [Pubmed abstract] This work was supported by The Arthritis Society. |
Influenza: Identifying Risk of Severe Illness
The researchers found that blood levels of IL-6 were increased in patients that required critical care. IL-6 was also elevated when measured in a mouse model of the disease. They examined a mouse strain that lacks IL-6 and determined that while heightened IL-6 is associated with severe illness, lowering levels of IL-6 does not lead to improved outcome. In commenting on the importance of these results, Dr. Kelvin stresses that “Monitoring levels of IL-6 in patients with this subtype of influenza could serve as a possible readout of risk for severe illness, and may lead to improved care plans and outcomes.” Interleukin-6 is a potential biomarker for severe pandemic H1N1 influenza A infection. Paquette SG, Banner D, Zhao Z, Fang Y, Huang SS, Le?n AJ, Ng DC, Almansa R, Martin-Loeches I, Ramirez P, Socias L, Loza A, Blanco J, Sansonetti P, Rello J, Andaluz D, Shum B, Rubino S, de Lejarazu RO, Tran D, Delogu G, Fadda G, Krajden S, Rubin BB, Bermejo-Martin JF, Kelvin AA, Kelvin DJ. PLoS One. 2012 June 5. [Pubmed abstract] This work was supported by the Li Ka Shing Foundation and Immune Diagnostics & Research. |
 |
![]()
 Dr. Badru Moloo, Director of UHN’s Animal Resources Centre (ARC), is the recipient of the Tecniplast Veterinarian of the Year Award from the Canadian Association for Laboratory Animal Science (CALAS), a national association dedicated to providing high quality training and educational resources to animal care professionals across Canada. This award recognizes the efforts and dedication of a veterinarian within the CALAS organization who supports and encourages excellence in Laboratory Animal Science locally and nationally. CALAS awards were presented to recipients at its 51st Annual Meeting, held in Vancouver on June 3.
Dr. Badru Moloo, Director of UHN’s Animal Resources Centre (ARC), is the recipient of the Tecniplast Veterinarian of the Year Award from the Canadian Association for Laboratory Animal Science (CALAS), a national association dedicated to providing high quality training and educational resources to animal care professionals across Canada. This award recognizes the efforts and dedication of a veterinarian within the CALAS organization who supports and encourages excellence in Laboratory Animal Science locally and nationally. CALAS awards were presented to recipients at its 51st Annual Meeting, held in Vancouver on June 3.As ARC Director, Dr. Moloo supervises the logistical demands and needs of researchers using animal models at UHN and maintains the highest standards of ethical care.
UHN researchers will lead three of the 14 projects funded by FedDev Ontario. These include projects focused on novel therapies for deep brain stimulation to treat Alzheimer’s disease (Dr. Andres Lozano, TWRI), a home diagnostic tool for sleep apnea (Dr. Geoff Fernie, TRI) and a portable device for the detection of hydrocephalus (Dr. Kieran Murphy, TWRI). UHN is a formal collaborator on two additional projects involving the discovery of new methods to identify a swallowing disorder called dysphagia (Dr. Tom Chau, TRI) and KINARM, a robotic virtual-reality device for the assessment of brain injury (Dr. George Mochizuki, TRI).
Dr. Geoff Fernie, TRI Director, served as the feature research speaker. Also in attendance at the announcement were UHN President and CEO Dr. Robert Bell and Dr. Donald Stuss, President and Scientific Director, OBI. “This funding will support large-scale collaborative projects focused on the prevention, early diagnosis, treatment and management of brain disease,” said Minister Goodyear. “The innovative patient care and ground-breaking research will lead to job growth, healthier communities and a better quality of life for all Canadians and people around the world.”
 UHN’s 2011 Inventor of the Year Award was presented to OCI Scientist Dr. Aaron Schimmer. This award, sponsored through UHN’s Technology Development and Commercialization Office and presented by VP Research Dr. Christopher Paige, is in recognition of a UHN researcher who has made an outstanding and inventive contribution to patient-oriented biomedical research.
UHN’s 2011 Inventor of the Year Award was presented to OCI Scientist Dr. Aaron Schimmer. This award, sponsored through UHN’s Technology Development and Commercialization Office and presented by VP Research Dr. Christopher Paige, is in recognition of a UHN researcher who has made an outstanding and inventive contribution to patient-oriented biomedical research.Dr. Schimmer was acknowledged for his efforts in advancing therapeutics from the lab to the bedside clinic. Known drugs are screened by Dr. Schimmer’s research team to identify compounds that impact molecular targets responsible for cancer malignancies and thus have previously unrecognized anti-cancer activity. Through this approach, current drugs can be ‘repurposed’ and moved into clinical trials at a fraction of the time and resources typically needed for developing cancer medicine.
Fourteen patents have been applied for as a result of the successes of this research initiative. Dr. Schimmer’s work has led to new insights into molecular pathways and novel treatments. Congratulations Dr. Schimmer!
Feedback
Net Results EXPRESS is brought to you by UHN Research Communications. We hope you have enjoyed receiving this message. If you have any feedback, please email www@uhnresearch.ca.
To access archived issues of Net Results EXPRESS, visit uhnresearch.ca/news/netresultsexpress
Some images adapted from the image archives of stock.xchng.ca, Wikimedia Commons and Wikipedia. There is increasing evidence that many tumours are maintained by cancer-initiating cells. These cells have the ability to initiate the growth of cancer cells that make up the bulk of the tumour as well as have the capacity to self-renew to maintain itself. This ability to self-renew is suspected to be the reason why tumours can develop resistance to therapy. A recent study from OCI Scientist Dr.
There is increasing evidence that many tumours are maintained by cancer-initiating cells. These cells have the ability to initiate the growth of cancer cells that make up the bulk of the tumour as well as have the capacity to self-renew to maintain itself. This ability to self-renew is suspected to be the reason why tumours can develop resistance to therapy. A recent study from OCI Scientist Dr.  Heart failure is a leading global public health issue that results in increased hospital care and high mortality rates. Diagnosis by an emergency care physician who may not have a full range of prognostic tools available could result in the hospitalization of low-risk patients and the potential discharge of high-risk patients, who could die as a result. In an effort to assist emergency care physicians treating patients with symptoms of heart failure, TGRI Scientist Dr.
Heart failure is a leading global public health issue that results in increased hospital care and high mortality rates. Diagnosis by an emergency care physician who may not have a full range of prognostic tools available could result in the hospitalization of low-risk patients and the potential discharge of high-risk patients, who could die as a result. In an effort to assist emergency care physicians treating patients with symptoms of heart failure, TGRI Scientist Dr.  More than 30 million genetic variations account for the diversity in DNA sequences in the human population. Thousands have so far been identified as risk factors for diseases including cancer. However, the mechanisms through which they impact disease development remain, for the most part, unknown. This stems from the fact that most disease-associated genetic variations lie outside of the gene itself. OCI Scientist Dr.
More than 30 million genetic variations account for the diversity in DNA sequences in the human population. Thousands have so far been identified as risk factors for diseases including cancer. However, the mechanisms through which they impact disease development remain, for the most part, unknown. This stems from the fact that most disease-associated genetic variations lie outside of the gene itself. OCI Scientist Dr.  Chronic alcohol abuse causes a number of negative health effects, including pancreatitis—an inflammation of the pancreas. However, alcohol may not simply be a direct culprit, as TWRI Affiliate Scientist Dr.
Chronic alcohol abuse causes a number of negative health effects, including pancreatitis—an inflammation of the pancreas. However, alcohol may not simply be a direct culprit, as TWRI Affiliate Scientist Dr. 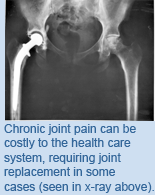 Arthritis comprises a large group of disorders that affect joints, ligaments, tendons, bones and other components of the musculoskeletal system. It is characterized by inflammation of these regions, causing pain and difficulty in movement. It often affects older people and with an aging population it is one of the most commonly reported chronic conditions. A recent survey of the health of the Canadian population by Statistics Canada found that 16% reported having arthritis—but it was the number of people reporting joint pain without the presence of arthritis that caught the attention of TWRI Senior Scientist Dr.
Arthritis comprises a large group of disorders that affect joints, ligaments, tendons, bones and other components of the musculoskeletal system. It is characterized by inflammation of these regions, causing pain and difficulty in movement. It often affects older people and with an aging population it is one of the most commonly reported chronic conditions. A recent survey of the health of the Canadian population by Statistics Canada found that 16% reported having arthritis—but it was the number of people reporting joint pain without the presence of arthritis that caught the attention of TWRI Senior Scientist Dr.  The 2009 swine flu outbreak was caused by a newly emerging subtype of the influenza virus, known as pandemic H1N1 influenza A (H1N1pdm). Symptoms caused by H1N1pdm range from fever and sore throat to more severe cases of acute respiratory distress syndrome (ARDS), in which inflammation of the lungs can eventually lead to organ failure and death. Currently, there is no way for physicians to identify which patients with H1N1pdm will become seriously ill. In order to investigate differences in how the immune system responds to influenza infection, TGRI Senior Scientist Dr.
The 2009 swine flu outbreak was caused by a newly emerging subtype of the influenza virus, known as pandemic H1N1 influenza A (H1N1pdm). Symptoms caused by H1N1pdm range from fever and sore throat to more severe cases of acute respiratory distress syndrome (ARDS), in which inflammation of the lungs can eventually lead to organ failure and death. Currently, there is no way for physicians to identify which patients with H1N1pdm will become seriously ill. In order to investigate differences in how the immune system responds to influenza infection, TGRI Senior Scientist Dr.