
July 2011
Welcome to Net Results EXPRESS
Net Results EXPRESS (NRx) is an award-winning, monthly e-newsletter highlighting medical and scientific breakthroughs, major grants and honours awarded, and other research-related events at UHN. Through NRx you can read about ongoing research at our four research institutes, the Ontario Cancer Institute (OCI), the Toronto General Research Institute (TGRI), the Toronto Western Research Institute (TWRI) and the Toronto Rehabilitation Institute (TRI). We hope you will find this newsletter informative and helpful. If you have feedback or questions, please contact www@uhnresearch.ca. Christopher J. Paige, PhD, FCAHS |
UHN's New Office of Research Trainees
To lead this initiative, OCI Senior Scientist Dr. Linda Penn has been appointed as the new Director of the Office of Research Trainees. Having a strong track-record in the area of research training, Dr. Penn brings outstanding experience in the mentorship of trainees at all levels of their academic journey. In her own lab, she has trained 25 graduate students and 21 post-doctoral fellows. Most have held scholarship awards, published manuscript and review articles and have pursued full scientific careers in academia, medicine and/or industry. Her mentorship experience also extends outside of the lab through her role as Division Head of Cancer Genomics and Proteomics, and Head of Graduate Admissions, in the Department of Medical Biophysics, University of Toronto. The purpose of UHN’s ORT is:
The ORT will complement existing trainee committees within each Research Institute by working alongside representatives to ensure communication is transferred thoroughly throughout the organization. The ORT is managed by the research training committee, which includes the principal investigators and trainee representatives from within each research institute. Dr. Penn is supported by Priscilla De Luca who is the coordinator of the Office of Research Trainees. Please feel free to visit the ORT office located at: Princess Margaret Hospital |
Stem Cells: Isolation of the Purest Blood Stem Cells
The research team examined blood-forming cells and found a protein called CD49f expressed specifically on the surface of human HSCs. Using a technique known as flow cytometry, a process that discriminates cells based on cell surface proteins, they isolated cells expressing CD49f (CD49f+ cells) and evaluated their capacity to develop into the different types of cells that form a blood system. They transplanted a single CD49f+ cell into a mouse lacking an immune system and temporally monitored how human blood cells developed in these mice. They demonstrated that these cells were capable of developing into all the cell types of the blood system and that they were self-renewing, allowing them to function in the body over the long-term. These findings are a major step towards generating sufficient quantities of stem cells to enable greater clinical use and this helps to move us closer to realizing the promise of regenerative medicine for patients. "This discovery also means that we now have an increasingly detailed road map of the human blood development system," says Dr. Dick. "With this isolation technique in place, stem cell scientists can start characterizing the core properties of these cells that distinguish them from all other blood cell types." Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Notta F, Doulatov S, Laurenti E, Poeppl A, Jurisica I, Dick JE. Science. 2011 Jul 8. [Pubmed abstract] This work was supported by grants from the Canadian Institutes of Health Research, the Terry Fox Foundation, Genome Canada through the Ontario Genomics Institute, Ontario Institute for Cancer Research, the Ontario Ministry of Health and Long-Term Care and the Canada Research Chairs Program. |
Clinical Trials: Assessing the Effects of Unpublished Data
The group charted the progress of 709 phase III trials presented at annual meetings of the American Society of Clinical Oncology between 1989 and 2003. Of these trials, 66 (9.3%) remain unpublished and 94 (13%) had delayed publication of more than 5 years. The clinical importance of these unpublished trials was assessed by a group of oncology experts. They determined that 70% of unpublished studies addressed an important question in the field of cancer and 59% of the studies might have had a clinical impact had they been published promptly. "Failure to publish phase III clinical trials with negative results could lead to significant overestimation of treatment benefits and could adversely affect clinical practice," says Dr. Krzyzanowska. "This practice violates an agreement with patients, institutions and funding agencies and can have serious ramifications for future clinical trials." Compendium of unpublished phase III trials in oncology: characteristics and impact on clinical practice. Tam VC, Tannock IF, Massey C, Rauw J, Krzyzanowska MK. Journal of Clinical Oncology. 2011 Jul 11. [Pubmed abstract]
|
Gastroenterology: New Insights into Blood Glucose Regulation
After a period of re-feeding, rats that were given a drug that inhibits the actions of PKC in the duodenum (the first section of the small intestine) had significantly increased blood glucose levels. This drug was believed to have interfered with the correct mechanisms involved in detecting nutrients in the gut, leading to improper glucose regulation. Conversely, activation of a form of PKC (PKC-d) in the duodenum decreased blood glucose levels. "Our research reveals a new glucose-regulatory role of duodenal PKC-d and strengthens the hypothesis that the gut has an early role in sensing nutrients available for uptake to trigger the brain to react to this nutrient imbalance," says Dr. Lam. "We believe that activation of this system in the duodenum could lower blood glucose levels in diabetes and obesity." Duodenal mucosal protein kinase C-d regulates glucose production in rats. Kokorovic A, Cheung GWC, Breen DM, Chari M, Lam CKL, Lam TKT. Gastroenterology. 2011 Jun 22. [Pubmed abstract] This work was supported by a research grant from the Canadian Institutes of Health Research. |
Blood: New Directions for Disease Treatment
Patients were treated with decitabine–a drug known to cause expression of silenced genes–for 12 weeks and monitored blood levels for beneficial and toxic effects. The group found that decitabine led to an overall increase in hemoglobin levels and red blood cell production. The drug was also found to be nontoxic. Previous therapies for ß-thalassemia involved frequent blood transfusions or drugs with mutagenic properties. "By its nature, decitabine is nonmutagenic and appeared safe for patients, but this is a pilot study and further studies are anticipated," says Dr. Olivieri. "We now have a potential new method of treating ß-thalassemia that is distinct from conventional therapy and could offer more options for future patients." A pilot study of subcutaneous decitabine in ß-thalassemia intermedia. Olivieri NF, Saunthararajah Y, Thayalasuthan V, Kwiatkowski J, Ware RE, Kuypers FA, Kim HY, Trachtenberg FL, Vichinsky EP. Blood. Jun 23. [Pubmed Abstract] This work was supported by a grant from the National Institutes of Health – National Heart Lung and Blood Institute. |
Stroke: Understanding the Short-Term Outcomes for Artery Blockage
The long-term effects of an internal carotid artery (ICA) occlusion, a condition where the main artery supplying blood to the brain is blocked, can range from a lack of symptoms to severe stroke. However, there is limited knowledge of the short-term outcomes of ICA occlusion. A study led by TWRI Clinician Investigator Dr. Leanne Casaubon and TGRI’s Dr. Moira Kapral examined the immediate consequences of ICA occlusion compared with other situations affecting blood flow through the ICA, including, the constriction of the carotid artery (ICA stenosis). This multicentre study examined short-term outcomes–including stroke, worsening of neurological function, seizure and death–in patients with ICA occlusion versus severe, moderate or mild ICA stenosis. They determined that patients with ICA occlusion are more likely to suffer from poor recovery of physical function, early recurrent stroke and in-hospital death when compared to patients with stenosis. The study recommends a change in treatment for patients with ICA occlusions. "We believe that more aggressive management may be necessary," says Dr. Casaubon. "Earlier and more intensive therapy may be warranted to prevent stroke. Revascularization, a procedure where blood flow is re-routed around the blocked area, may also benefit patients." Short-term outcomes after symptomatic internal carotid artery occlusion. Burke MJ, Vergouwen MD, Fang J, Swartz RH, Kapral MK, Silver FL, Casaubon LK; on behalf of the Investigators of the Registry of the Canadian Stroke Network. Stroke. 2011 Jul 14. [Pubmed abstract] This work was supported by grants from the Ontario Ministry of Health and Long-Term Care, the Niels Stensen Foundation, the Heart and Stroke Foundation, the Canadian Institutes of Health Research, the Canadian Stroke Network and the University Health Network Women’s Health Program. |
 |
![]()
| UHN Researcher Named to the Order of Canada |
Appointees to the Order of Canada were announced by the Governor General of Canada, the Right Honourable David Johnston on Thursday June 30, 2011. The Order of Canada, one of our country’s highest civilian honours, was established in 1967, during Canada’s centennial year, to recognize a lifetime of outstanding achievement, dedication to community and service to the nation. |
| UHN Captures New Funding |
Funding for this project comes from the Ontario Research Fund – Research Excellence Program Round 5 competition. This $65M funding opportunity by MRI awarded 26 projects across the province. For more information please visit mri.gov.on.ca. |
| Dr. Moulton Receives Early Researcher Award |
The award will support Dr. Moulton’s research into medical error prevention and patient safety. Her work aims to explore the causes of self-regulatory failures of surgeon error, with an emphasis on psychosocial components. |
| TGRI Researcher Lauded |
Congratulations to TGRI Senior Scientist Dr. Peter Liu for receiving the Canadian Cardiovascular Society (CSS) Annual Achievement Award. Dr. Liu received this award for his contributions to the field of cardiovascular research. Previous CCS Achievement Award winners include TGRI members Drs. Tirone David and Peter McLaughlin. |
| Dr. Gallie Named Researcher of the Month |
CHR promotes health research issues, scientific processes and their impact to the public. Previous UHN Researcher of the Month awardees include Drs. Tak Mak and Katherine Siminovitch. |
| UHN 2010 Research Report Honoured with Award |
The report competed in the “Health Care-Providers and Services” category and received a total score of 95 out of a maximum 100 points. “This year’s annual report for UHN proves to be remarkable in light of tremendous competition,” said Christine Kennedy, LACP Managing Director. Other comments included “…we congratulate the entire team involved with this year’s work. We classify this entry as being among the best annual reports within its industry this year.” To view the 2010 report, please click here. |
Feedback
Net Results EXPRESS is brought to you by UHN Research Communications. We hope you have enjoyed receiving this message. If you have any feedback, please email www@uhnresearch.ca.
To access archived issues of Net Results EXPRESS, visit uhnresearch.ca/news/netresultsexpress
Some images adapted from the image archives of stock.xchng.ca.
 UHN is pleased to announce the launch of the new Office of Research Trainees (ORT). As a member of the Toronto Academic Health Science Network and home to more than 900 research students and fellows, UHN is committed to providing an excellent environment for the scholarly development of our students and post-doctoral fellows.
UHN is pleased to announce the launch of the new Office of Research Trainees (ORT). As a member of the Toronto Academic Health Science Network and home to more than 900 research students and fellows, UHN is committed to providing an excellent environment for the scholarly development of our students and post-doctoral fellows.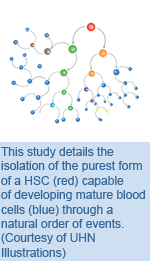 Hematopoietic stem cells (HSCs) are solely responsible for lifelong blood production, but they are very rare making them difficult to isolate. In the current study, published in Science and led by the McEwen Centre for Regenerative Medicine and UHN Senior Scientist Dr.
Hematopoietic stem cells (HSCs) are solely responsible for lifelong blood production, but they are very rare making them difficult to isolate. In the current study, published in Science and led by the McEwen Centre for Regenerative Medicine and UHN Senior Scientist Dr.  Clinical trials examining the therapeutic effect and potential toxicity of a new drug on a large sample population, known as phase III clinical trials, must be performed to show evidence of effectiveness of a drug prior to being issued approval by federal agencies like Health Canada. However, some groups do not publish the results of these phase III trials, particularly when the results are negative, which could potentially deprive doctors and patients of vital information. A study by OCI Investigator Dr.
Clinical trials examining the therapeutic effect and potential toxicity of a new drug on a large sample population, known as phase III clinical trials, must be performed to show evidence of effectiveness of a drug prior to being issued approval by federal agencies like Health Canada. However, some groups do not publish the results of these phase III trials, particularly when the results are negative, which could potentially deprive doctors and patients of vital information. A study by OCI Investigator Dr. 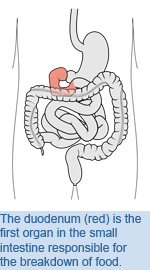 TGRI's Dr.
TGRI's Dr. 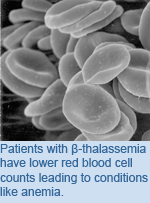 TGRI Senior Scientist Dr.
TGRI Senior Scientist Dr.  UHN congratulates Dr.
UHN congratulates Dr.  The Ontario Ministry of Research and Innovation (MRI) has awarded TWRI’s Dr.
The Ontario Ministry of Research and Innovation (MRI) has awarded TWRI’s Dr.  Congratulations to Dr. Carol-Anne Moulton, Scientist at the Wilson Centre and Surgeon in the Division of General Surgery and Surgical Oncology, who has received an Early Researcher Award (ERA) from the Ontario Ministry of Research & Innovation. The ERA program helps promising, recently-appointed Ontario researchers build their research teams of undergraduates, graduate students, post-doctoral fellows, research assistants, associates, and technicians.
Congratulations to Dr. Carol-Anne Moulton, Scientist at the Wilson Centre and Surgeon in the Division of General Surgery and Surgical Oncology, who has received an Early Researcher Award (ERA) from the Ontario Ministry of Research & Innovation. The ERA program helps promising, recently-appointed Ontario researchers build their research teams of undergraduates, graduate students, post-doctoral fellows, research assistants, associates, and technicians. Dr.
Dr.  The 2010 UHN Research Report, entitled Driving Health Innovation: Research Report 2010, was recognized by the League of American Communications Professionals (LACP) with a Bronze Award in the Annual Report Competition - the 2010 Vision Awards. LACP received more than 5000 entries in this year’s competition, representing upwards of two-dozen countries and 800 entities.
The 2010 UHN Research Report, entitled Driving Health Innovation: Research Report 2010, was recognized by the League of American Communications Professionals (LACP) with a Bronze Award in the Annual Report Competition - the 2010 Vision Awards. LACP received more than 5000 entries in this year’s competition, representing upwards of two-dozen countries and 800 entities.